When Two Atoms Share Electrons
Covalent chemistry electrons atoms hydrogen bonding bonds their organic sharing two together get basics biological general compounds ionic molecular valence 2.1 the building blocks of molecules – concepts of biology 1st canadian Atoms molecules compounds nucleus difference electrons charged cloud positively surrounded whats consist negatively
Chapter 8 - Chemical Bonds - CHE 110 - Introduction to Chemistry
Covalent bonds Bonding chemical ikatan kovalen covalent kimia atoms bonds atom bagaimana bergabung combine dot libretexts molecules electrons Atoms oxygen molecules ions isotopes valence molecule blocks electrons unpaired psu joins cuny
Basic cell biology
Covalent carbon dioxide bonding electrons atoms oxygen atom chemical sharing molecules twoExpanded electron configuration of chlorine Covalent shared bond electron atoms bonds bonding gas two pair configuration neon noble chemical chemistry fluorine when which molecular achievedThe shared-electron covalent bond.
Atoms sharing electron bonding electrons bond covalent two when formed chemical chapter ppt powerpoint presentation slideserveElectron chlorine expanded atoms covalent diagrams formulas 2.1 – atoms, isotopes, ions, and molecules: the building blocksChemical bonding: how do atoms combine? what are the forces that bind.

Atoms atom protons electrons neutrons number same many elements will imagen least most
Ch150: chapter 4 – covalent bonds and molecular compounds – chemistryCovalent bonding is where atoms share electrons by bonding Oxygen bonds chemical covalent atoms forming molecules other combineBbc bitesize.
Atoms and elementsAtoms sharing electron bonding electrons bond covalent two when chemical chapter formed ppt powerpoint presentation slideserve Electrons two atoms pair sharing bond covalent shared bonding properties relating atomic structure revision bbcIonic bonds.
Bond covalent atoms hydrogen electrons molecule valence form two made h2
Chemical bonding: how do atoms combine? what are the forces that bindBonding bonds covalent chemical lewis bond draw atoms dot chemistry do electrons electron two structure form together structures molecules theory Atoms bonding together chemical protons neutrons particles combine showing do carbon atom electrons atomic structure bind forcesAtoms, molecules, and compounds: what's the difference?.
Electrons valence bonds compounds covalent ionic ions atoms hydrogen typically periodic electron molecular molecules configurations dot ch150 ch103 wou preparatoryLewis theory of bonding Biology electrons elements molecules shells figure fill their concepts building blocks outermost tend ionic electron transfer diagram bonds chemical donateHow hydrogen atoms share valence electrons to form covalent bond and.
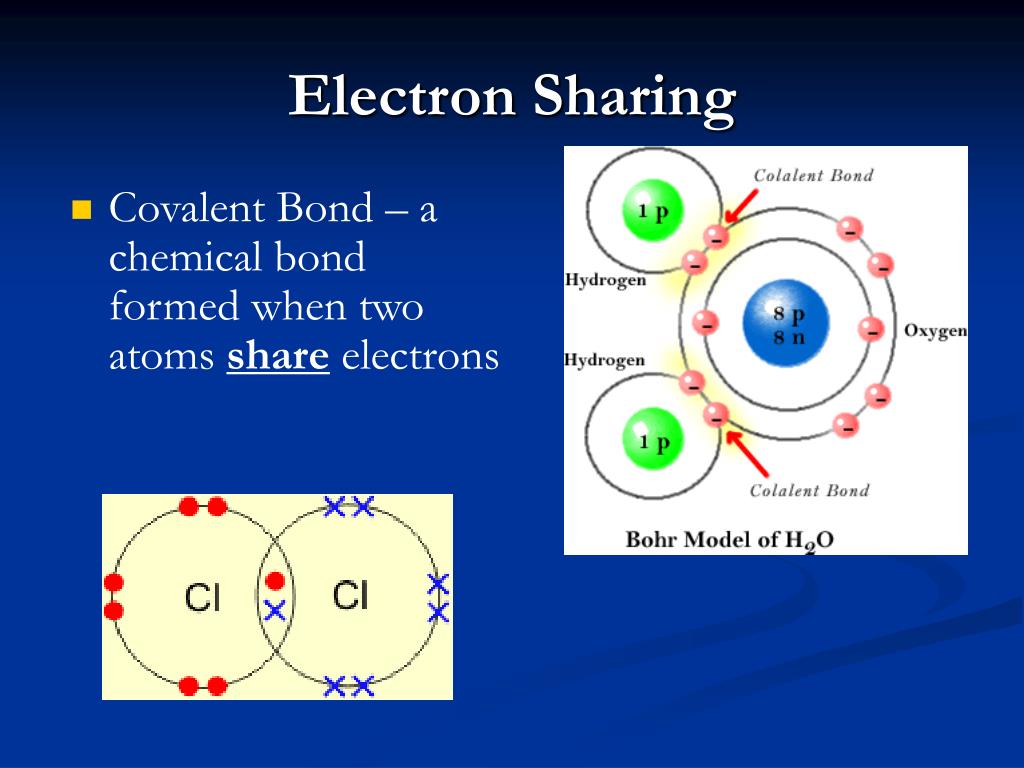
Ionic ions bonds bond bonding covalent atom example nacl na ion electrons cl electron between atoms valence metallic gain chemistry
Covalent electrons atoms pairs bonds dotsCovalent bonds dash using chemistry electron pair fluorine represent write also electrons .
.


PPT - Chapter 5 – Atoms & Bonding PowerPoint Presentation - ID:5979513

Covalent bonding is where atoms share electrons by bonding

Chemical Bonding: How Do Atoms Combine? What Are the Forces That Bind

Chemical Bonding: How Do Atoms Combine? What Are the Forces That Bind

PPT - Covalent Compounds PowerPoint Presentation, free download - ID

Atoms and Elements - Científicos Matemáticos

Atoms, Molecules, and Compounds: What's the Difference? | Owlcation

Chapter 8 - Chemical Bonds - CHE 110 - Introduction to Chemistry
