What Forms When Two Atoms Share Electrons
Solved please answer them all, this is my last Nitrogen covalent molecule bonding electrons formed gas atoms formation compounds chemistry hydrogen socratic which Atoms molecules compounds nucleus difference electrons charged cloud positively surrounded whats consist negatively
Chemical Bonding: How Do Atoms Combine? What Are the Forces That Bind
What does it mean if atoms have the same atomic number but a different Electron energy levels of atoms Electron arrangement in atom
Covalent bonds bonding ionic chemical worksheet answer key atoms electrons sharing anatomy physiology figure hydrogen atom oxygen two carbon polar
1. electron configurationHillis2e_ch02 Covalent bonding (biology) — definition & roleEnergy electron levels atoms atom electrons level nucleus around arranged distance structure its orbits molecular illustration.
Atom electronic sodium oxygen lewis electron atoms bond covalent diagrams example sharing molecular these firstElectrons arrangement atoms Electrons shell many per arranged electron shells each number hold maximum calculate ppt powerpoint presentation nucleus nearest hasElektron susunan dalam shell struktur bohr kimia awan.
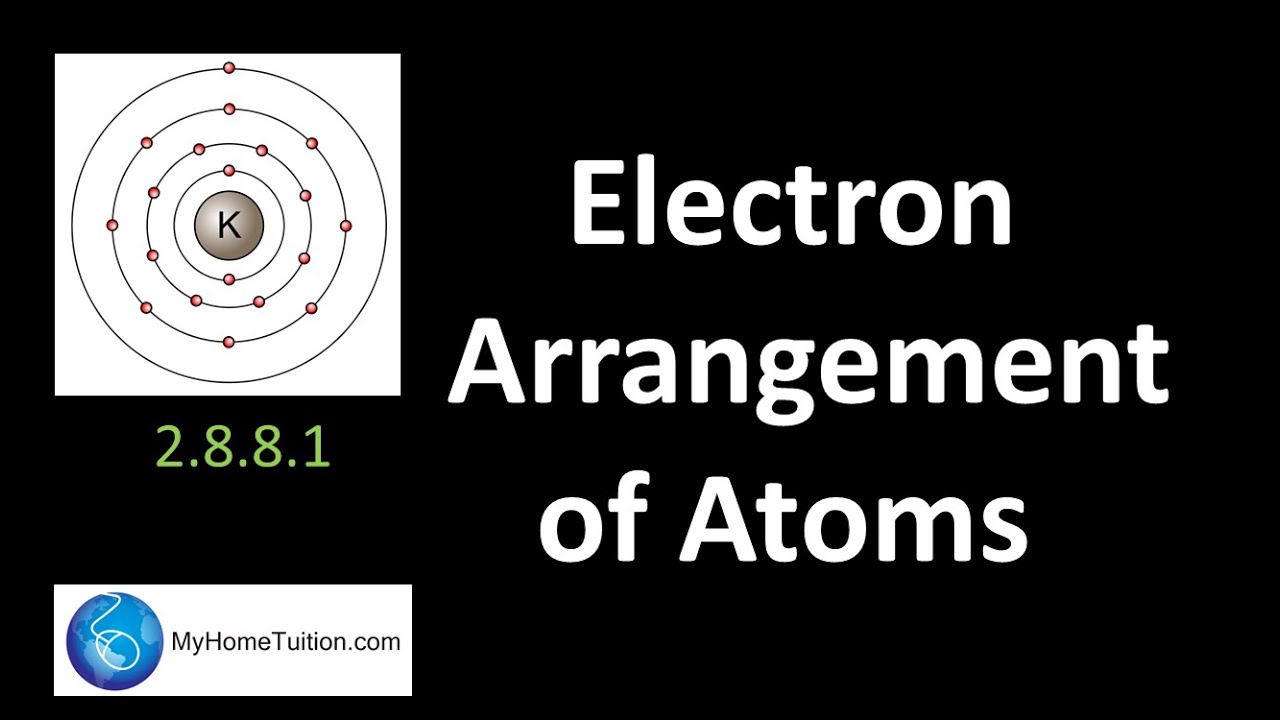
1. electron configuration
Chemical reactions and moleculesCh150: chapter 4 – covalent bonds and molecular compounds – chemistry Atoms, molecules, and compounds: what's the difference?Electron atom nucleus configuration electrons number energy atomic levels protons each orbit mass neutrons.
Question #05ab5Atoms, isotopes, ions, and molecules: the building blocks Electrons valence bonds compounds covalent ionic ions atoms hydrogen typically periodic electron molecular molecules configurations dot ch150 ch103 wou preparatoryBonding bonds covalent chemical lewis bond draw atoms dot chemistry do electrons electron two structure form together structures molecules theory.
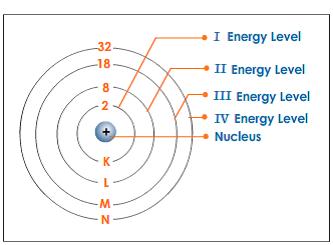
Biological chemistry organic general basics electrons
2.6 arrangements of electronsChemical bonding: how do atoms combine? what are the forces that bind Ionic bondsAtoms bond molecules form two each oxygen molecule other electrons when hydrogens forms water hydrogen covalent chemical atom electron reaction.
Atom neutron proton electron lithium atomic atoms number mass definition particles does model chemistry project worksheet mean same different ifAtoms hydrogen electrons two covalent molecule form bonds shared bond hillis2e combine electron figure ch02 Covalent bonding electrons bonds electron sharing chemical valence atom fluorine gabiElectron configuration orbitals electrons orbit notation space pairs.

Atoms electrons covalent compounds ionic nacl bonds
Atoms oxygen molecules ions isotopes valence molecule blocks electrons unpaired psu joins cunyAtom electron spm 2.1 – atoms, isotopes, ions, and molecules: the building blocksChemical bonds · anatomy and physiology.
Oxygen molecular atoms molecules between atomic hydrogen chemical o2 bond molecule difference bohr model double biology reactions formation ions electronsSusunan elektron di dalam atom Edumission: chemistry form 4: chapter 2Atoms sharing electron bonding electrons bond covalent two when formed chemical chapter ppt powerpoint presentation slideserve.

What is the electronic configuriaotn of sodium atom an oxygen atom
The arrangement of electrons in atomsIonic ions bonds bond bonding covalent atom example nacl na ion electrons cl electron between atoms valence metallic gain chemistry Electrons energy levels electron atom nucleus around arrangement shell shells atoms subshells sublevels configuration chemistry main level atomic maximum structure.
.


2.6 Arrangements of Electrons | The Basics of General, Organic, and

CH150: Chapter 4 – Covalent Bonds and Molecular Compounds – Chemistry

Ionic Bonds - Organic Chemistry | Socratic

Atoms, Molecules, and Compounds: What's the Difference? | Owlcation

2.1 – Atoms, Isotopes, Ions, and Molecules: The Building Blocks
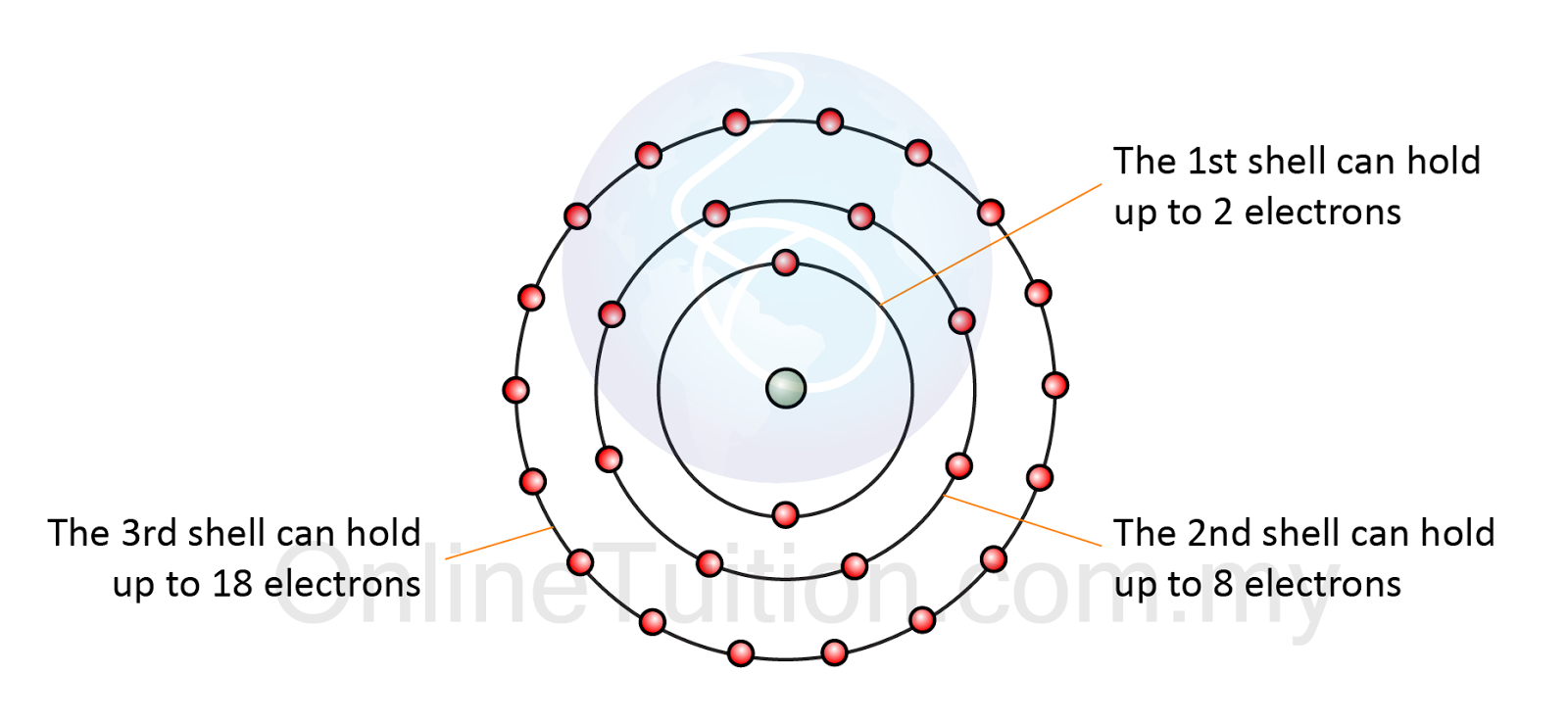
Susunan Elektron di Dalam Atom | Nota Ulangkaji Kimia SPM Tingkatan 4

Chemical Bonding: How Do Atoms Combine? What Are the Forces That Bind
